The term "toughener" describes a chemical that can make the adhesive film more flexible. The elongation and brittleness of several thermosetting resin adhesives, such as epoxy resin, phenolic resin, and unsaturated polyester resin adhesive, are low after curing. The adhesive layer is easily cracked when the bonding component is subjected to external stress, and it is not fatigue resistant, making it unsuitable for structural bonding.
In order to enhance bearing strength, hardness, and brittleness, we must minimize all three. A toughening agent is any compound that may improve toughness and decrease brittleness without impacting the adhesive's other essential qualities. Tougheners often have active groups that can interact chemically with the resin. After curing, they are not entirely compatible, and occasionally they must separate phases to provide a more perfect toughening effect, causing the heat distortion temperature to either stay the same or slightly decrease. Significant improvements have been made to the impact resistance.
Although the brittleness may also be decreased by adding certain low-molecular liquids or plasticizers to the resin, the stiffness, strength, and thermal distortion temperature are significantly decreased, which makes them unsuitable for structural bonding. Plasticizers and Tougheners so differ greatly from one another.
While the heat distortion temperature has reduced, some linear polymer compounds can be miscible with the resin and include active groups that can take part in the resin's curing reaction and increase the material's elongation at break and impact strength.The name of this chemical is softening. Because they may be converted into structural adhesives when correctly matched with resin, Flexibizer, liquid polysulfide rubber, and liquid nitrile rubber are frequently utilized; hence, the softener is also referred to as a toughening agent. Although the ideas of softening and toughening are distinct and connected, it can be challenging to make a clear distinction between the two. Toughening and softening are distinct in theory. The process of toughening does not cause the material to become softer overall; rather, it converts the homogeneous, cured epoxy resin system into a multiphase system by causing the toughening agent to aggregate into spherical particles.
hening agent causes the epoxy resin to form spherical particles. When the cross-linked network of the resin forms the dispersed phase, the anti-cracking performance quickly changes and the fracture toughness is greatly increased, while the loss of mechanical characteristics and heat resistance is minimal.
강화 메커니즘
Different toughening mechanisms are used with various types of tougheners. When liquid polysulfide rubber reacts with epoxy resin, it can introduce a portion of a flexible chain segment, lower epoxy resin's modulus, increase toughness, but at the expense of heat resistance.The bonding strength reduces instead of increasing when liquid nitrile rubber is used as a toughening agent for epoxy resin; only the medium- and high-temperature curing systems have observable toughening and bonding effects. Carboxyl-terminated liquid nitrile rubber toughened epoxy resin forms a "sea-island structure" that may absorb impact energy without compromising heat resistance by being compatible before curing and separating after curing. The T-99 multi-functional epoxy curing agent cures epoxy resin in a way that introduces flexible chain segments into the cross-linked structure, which, in essence, does not result in a phase-separated structure and instead increases toughness without reducing heat resistance.
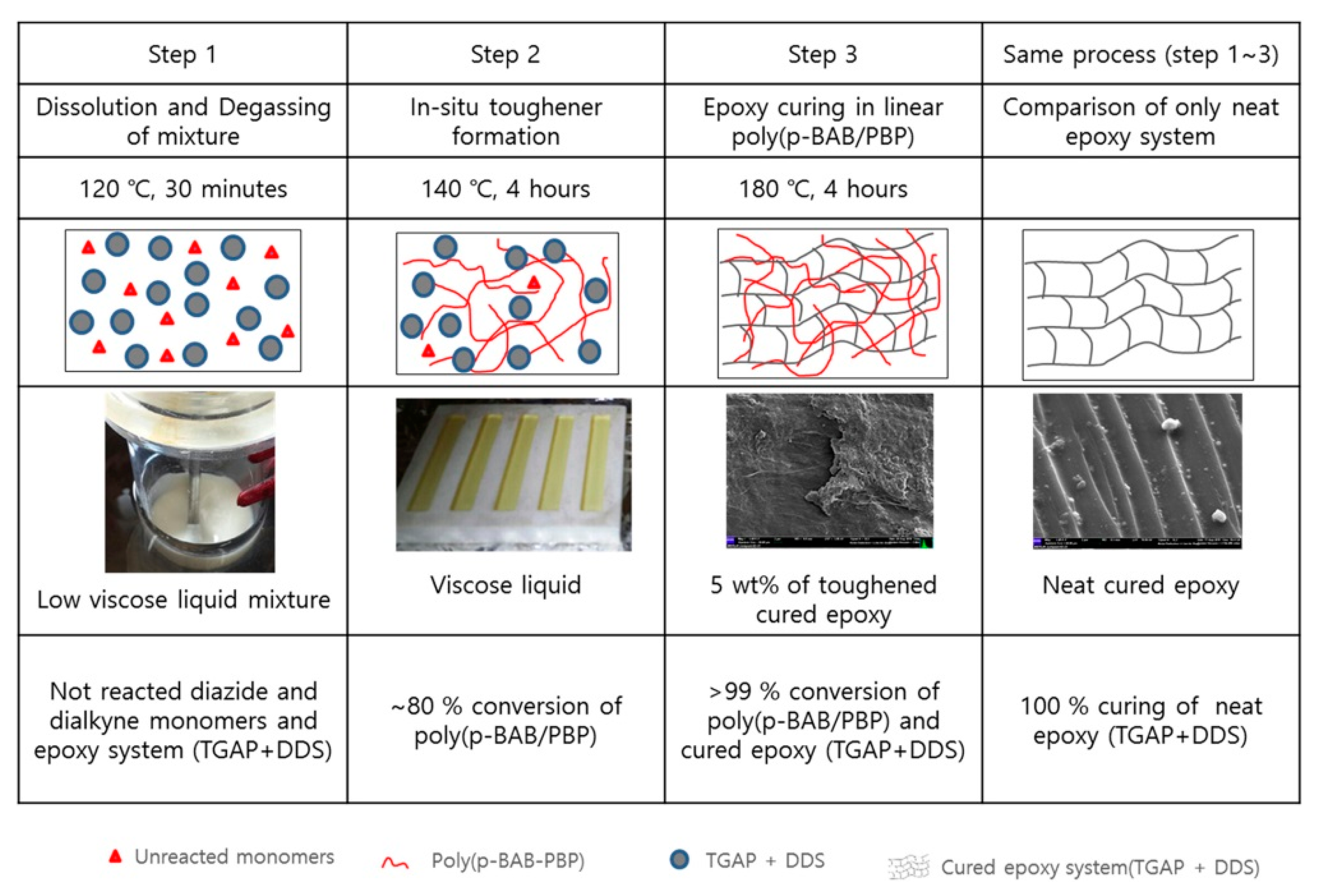
The semi-interpenetrating network polymer created by the thermoplastic resin flowing continuously through the epoxy resin network increases the toughness of the hardened epoxy resin.
Nanoparticles range in size from 1 to 100 nm, have a sizable specific surface area, and have exceptionally high levels of surface atom unsaturation, which results in a sizable surface activity. The epoxy group has a far bigger impact on the nanoparticle interface than the Van der Waals force, which can start microcracks and absorb energy. Both nano-SiO2 and nano-clay have the ability to start and stop silver crazes.The nanoparticles have a high degree of stiffness at the same time, so when the crack widens, they will be deflected or deflected and the energy will be absorbed to achieve the goal of toughening. Additionally, the resin and nanoparticles work well together to strengthen the matrix's capacity to spread and absorb impact energy, increasing toughness.











