Polypropylene (PP), one of the five all-purpose polymers, is widely utilized in many facets of society. The flammability of PP, however, also restricts its range of applications and prevents the advancement of PP materials. As a result, the modification of PP Sex to make it flame retardant has always drawn interest.
Next, let’s examine the polymer materials represented by PP’s combustion process and flame retardant mechanism, as well as the stock of flame retardant PP and its potential for use in the packaging industry.
Process and Mechanism of Polymer Materials’ Combustion
01 The burning process
Polymer compositions with carbon, hydrogen, oxygen, and other elements in the molecular chain are referred to as polymer materials. Most polymers can catch fire.
Special phenomena, such as melting softening and volume changes, will manifest during the combustion of polymer materials since it is a mix of physical changes and chemical processes. Figure 1 depicts the combustion of polymer materials, which may be roughly broken down into the following three steps:
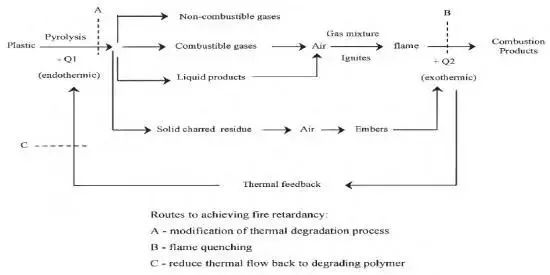
Fig.1 Schematic diagram of polymer combustion and flame retardancy
(1) The material will start to experience thermal breakdown processes as the temperature rises gradually, breaking the weaker links in the molecular chain. Small molecular gases, the majority of which are flammable, are gradually produced on the surface of the material as the thermal decomposition reaction of polymer materials continues and intensifies. These flammable small molecular gases are mixed with oxygen in the air to form flammable sexual mixed gas;
(2) The concentration of combustibles in the mixed gas on the surface of the polymer material rapidly rises as the breakdown reaction develops. Violent explosions will happen when the temperature and combustible gas content of the combined gas meet the necessary conditions for combustion. Chemical reaction causes the material’s surface to ignite fast;
(3) The explosive mixture of gas burns quickly and produces a lot of heat. In addition to spreading to the material’s bottom, the heat generated will also raise the temperature of the area around it. This will hasten the material’s decomposition, produce more flammable gas, and ultimately cause the combustion reaction to continue. As a result, it is possible to think of the combustion of polymer materials as a process of stepwise promotion and cyclic reaction.
PP has a low hydrocarbon oxygen index of 17.4, which makes it easily combustible, poorly flame-retardant, and heat-producing when burned. In addition, when it drips, it easily catches fire, endangering both people and property. It is vital to conduct research and produceflame retardant PPmaterials since the flammability of PP restricts its wider applicability in the field of electrical appliances.
02 Mechanism for containing flames
Chain reaction termination mechanisms, surface isolation mechanisms, and interrupted heat exchange mechanisms are approximately the two groups into which the flame retardant mechanisms fall.
(1) Mechanism for stopping a chain reaction When PP is burnt, it first breaks down into hydrocarbons and then, at a high temperature, thermally oxidizes further and fractures into free HO. The combustion may continue to burn due to the HO chain reaction, and the HO created during combustion is what puts an end to the chain reaction.
(2) Mechanism for surface insulation In addition to absorbing heat from the burning PP, the flame retardant also creates a solid compound on the material’s surface that serves as a barrier between the matrix and the air, preventing combustion.
(3) Disruption of the heat exchange system This mechanism refers to the ability of the flame retardant to absorb a significant quantity of combustion heat during the combustion process, resulting in a shortage of heat for the combustion reaction, which causes the flame retardant effect to be achieved by self-extinguishing.
Use of Flame Resistant PP in the Packaging Industry
With its low density, high transparency, tastelessness, ease of processing and molding, low cost, and lack of toxicity, PP plastic has a lot of potential applications in the packaging industry. However, these applications are constrained by the material’s flammability and poor high temperature resistance. As a result, several academics have focused their attention recently on researching PP packaging materials with strong flame retardancy.
1.Car battery case
One of the most crucial parts of modern energy vehicles are batteries. Additionally, the battery case—which is in charge of ensuring the security of batteries—is crucial. Insulation, impact resistance, corrosion resistance, and strong flame retardancy are all requirements for battery packing. Metal materials and sheet molding compound (SMC) components make up the majority of traditional battery packaging. However, some of these two materials need intricate molding techniques and have a high density, which affects how lightweight future energy vehicles may be. The attention of people has thus been drawn to PP materials with low density and strong impact resistance.
It is made using the melt blending process and contains PP resin as the matrix, an ammonium polyphosphate/triazine chemical system as a flame retardant, ethylene-octene copolymer, a propylene-based elastomer, and EPDM as a toughener. a PP material with flame-retardant qualities that is utilized in new-energy cars as the battery housing. This PP material provides great waterproofing capabilities, good sealing capabilities, and good impact resistance while keeping a low density. It has been produced in large quantities.
02 Packaging for parts
Aluminum oxide (Al2O3) and basic magnesium sulfate whisker (MHSH) were modified. The surfaces of both were then crosslinked with KH-550, and PP matrix and flame retardant were added by melt blending. The procedure was used to create a PP/MHSH/Al2O3/N-P composite material, which was then further processed into a film.
Among them, the nitrogen-phosphorus compound flame retardant can enhance the intumescent carbon layer by reacting with MHSH to produce magnesium phosphate salt, which has good thermal stability. This reaction can also promote the formation of intumescent carbon layer on PP matrix at high temperatures. It provides stability and skeletal support.
Al2O3 can be added to a material to increase its thermal conductivity, allowing the material’s interior heat to be swiftly transmitted to the surface where it can contribute to heat dissipation and increase heat resistance. The mechanical characteristics of the PP/MHSH/Al2O3/N-P composite film can be improved by the rigidity and mechanical strength of MHSH and Al2O3, which are both used as fillers. Because of its outstanding flame retardancy and strong mechanical strength, the PP/MHSH/Al2O3/N-P composite film broadens the range of applications for PP composite materials.











