01 Justification for toughening nylon
Nylon, also known by its technical name polyamide, is a popular polymer that may be used to create textiles or engineered plastics.
Over 80 years have passed since Dr. Carothers of DuPont developed nylon, which was first used in 1935. Beginning with the original nylon 6 and 66, a massive family has now developed, encompassing aliphatic, semi-aromatic, and aromatic nylon, with a minimum of 20 species in all. This A value is still increasing since new nylon monomers are being synthesized continuously.
Nylon 6 and 66 are the most widely utilized of the various members of the nylon family. Simple, inexpensive, user-friendly, and cost-effectiveness are the causes.
Benefits of Nylon
Nylon 6 and 66, the most used engineering plastic, provide a number of benefits, including:
High mechanical toughness
simple to process
excellent heat resistance
durable
able to withstand chemical reagents
self-lubricating
excellent flame resistance
Absence of nylon
Despite nylon’s excellent performance, there are two significant drawbacks:
significant water absorption
poor resistance to cold temperatures
Nylon is infamous in the industry for having inadequate resilience at low temperatures; around minus 20 to 30 degrees, it is as fragile as glass.
DuPont created a toughening compound to increase nylon’s low-temperature toughness and lessen its water absorption in order to address the drawback of nylon’s weak low-temperature toughness.
02 What are the nylon toughening agents?
concept evaluation
Many similar terms, including tougheners, impact modifiers, cold-resistant agents, and compatibilizers, are misunderstood when discussing nylon tougheners.
Why does nylon get brittle in cold weather? only because it is too challenging. In theory, it can be resolved by combining nylon with certain soft materials (the softness and hardness in this context can be represented by the material’s yield strength), i.e., by combining nylon with a polymer material whose yield strength is lower than nylon’s.
Tougheners, impact modifiers, and cold-resistant compounds are all names used to describe the toughening of nylon, although compatibilizers are fundamentally distinct from them in terms of structure and function (will be detailed in detail below).
What substances are suitable for use as tougheners?
The toughness of nylon can be increased as long as the yield strength is lower than that of nylon, although there is a presumption that they must be compatible in some way. So, to varying degrees, rubber, polyethylene, polypropylene, thermoplastic elastomers, plasticizers, and even water can increase nylon’s low-temperature toughness.
Rubber and thermoplastic elastomers are now the two materials used in industry the most to toughen nylon. But now the issue reappears. These two substances are completely incompatible with one another since they are mostly made of the non-polar atoms carbon and hydrogen, whereas nylon is a strongly polar substance.
How do you handle it? Simple: just make the non-polar toughener more polar, and you’re done. To alter rubber and thermoplastic elastomers traditionally, polar monomers like maleic anhydride (MAH), glycidyl methacrylate (GMA), itaconic acid (ITA), etc. are used. The monomer utilized by DuPont and the one that is most frequently used is MAH.
What tougheners are most frequently employed?
Maleic anhydride grafted ethylene-octene copolymer (POE-g-MAH) and ethylene-propylene-diene rubber (EPDM-g-MAH) are now the two most widely used nylon tougheners.
POE-g-MAH
The US-based DOW Chemical Company created POE, also known as ethylene-1-octene copolymer, a polyolefin elastomer with exceptional performance. It has a homogeneous short chain branching in the main chain and a limited molecular weight range.
POE has excellent aging resistance, strong ozone resistance, good solvent resistance, and high tear strength.
EPDM-g-MAH
The copolymer known as EPDM, also known by its technical name EPDM rubber, is made of ethylene, propylene, and a trace quantity of non-conjugated diene. Unsaturated double bonds are present in the side chain whereas saturated double bonds make up the main chain. Although it has outstanding weather and aging resistance, it cannot compare to POE with fully saturated segments in terms of strength.
Given that nylon materials have a substantially higher yield strength than POE and EPDM, this can greatly increase nylon’s toughness at low temperatures. Their polarity rises upon MAH graft alteration, which improves nylon compatibility.
03 Strengthening nylon
The nylon toughening principle
The C=C double bond and the acid anhydride are the two important groups in the MAH molecule. These two groups work together to achieve the objective of enhancing nylon’s toughness while carrying out their individual tasks in the toughening process.
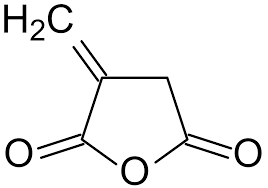
Let’s first discuss the function of the C=C double bond. The inert rubber or elastomer main chain must first be activated using a free radical initiator to create a free radical active site on the main chain. This allows the C=C double bond to be broken and grafted branches to be attached to the toughener’s main chain.
Next, let’s discuss the anhydride group. There is an anhydride group in the MAH that was grafted onto the toughening agent. When nylon and a toughening agent are combined, the anhydride will react with the amine group to create a complex that contains both the nylon and the toughening agent segments. This particular type of graft copolymer is referred to as a “compatibilizer” since it performs a completely different function from the toughener and has an entirely different structural makeup. The process may be illustrated in terms of chemical reactions using the following diagram.
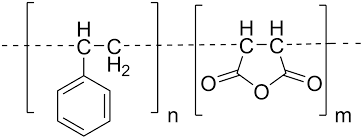
Compatibilizers are essential for making nylon more durable. It functions as a surfactant in nylon/toughener mixes because it comprises segments of both materials and has attraction for both nylon and toughener. It will therefore be uniformly dispersed in the nylon material after being preferentially distributed at the point where the two components of nylon and the toughening agent meet.
The size of the toughening agent’s scattered particles in nylon determines how toughened nylon is at low temperatures. The size of toughening agent particles decreases as compatibilizer concentration increases. The toughening agent particles are typically 200–500 nanometers in size when the nylon has been toughened to the super-tough level.
Process for toughening nylon
Melt blending in a twin-screw extruder is the most used technique for combining nylon with a toughening agent. The toughening agent and nylon interact chemically during the blending process to create a compatibilizer. The original Bit expansion is another name for this procedure.
When mixing nylon 6 with a toughening agent in an extruder, the temperature is typically between 220°C and 250°C, and the screw speed is used to control how long the process takes. When the time is cut too short, the outcome Poor nylon toughness results from insufficient compatibilizer concentration; if time is allowed to pass, enough compatibilizer can be created, but nylon will degrade to a greater extent.
04 Summary
Toughening is one of the most popular ways to modify nylon since pure nylon has relatively little toughness at low temperatures. POE-g-MAH and EPDM-g-MAH are the most widely utilized nylon toughening agents because of their effective toughening and practical operation. During the mixing process, the nylon and the toughening agent produce a compatibilizer in situ by using the reactivity of the MAH group on the toughening agent chain segment. The level of nylon toughening is dependent on the compatibilizer content and is connected to the toughening procedure. close.
When nylon is combined with a toughening agent, the material’s water absorption and low-temperature toughness may both be greatly enhanced, but the tensile strength is drastically decreased. The objective of research on nylon toughening has always been to achieve a balance between strength and toughness, whereby, as nylon’s toughness grows, its tensile strength remains the same or slightly declines.











